FDA Grants Accelerated Approval to ZEGFROVY, First Oral EGFR‑Exon‑20 Insertion NSCLC Therapy
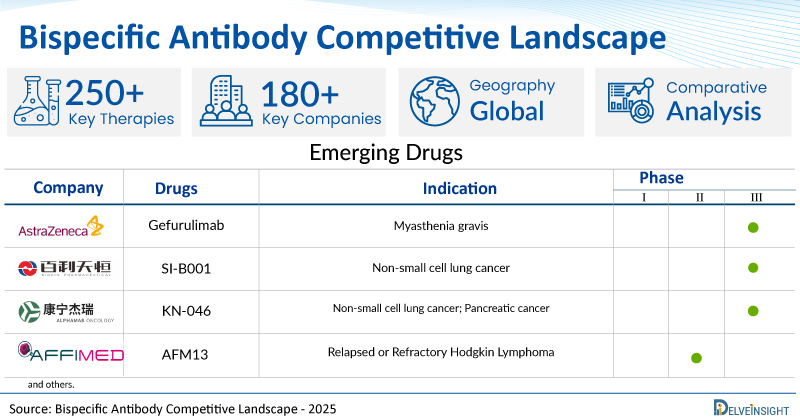
The U.S. Food and Drug Administration (FDA) has granted accelerated approval to ZEGFROVY (sunvozertinib), a novel oral therapy developed by Dizal, for the treatment of adults with locally advanced or metastatic non-small cell lung cancer (NSCLC) harboring …