Virtual Clinical Trials Market Revenue, Size, Share Report, 2030
Virtual Clinical Trials Market to Grow by USD 12.14 Billion During 2023-2030 | Featuring Clinical Ink, Dassault Systemes , IQVIA Holdings, Medable and Others
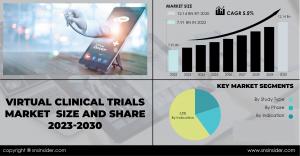
AUSTIN, TEXAS, UNITED STATES, April 18, 2024 /EINPresswire.com/ -- The virtual clinical trials market has been experiencing remarkable growth in recent years, driven by advancements in technology, increasing adoption of decentralized trial methodologies, and the need for more efficient and patient-centric approaches to clinical research. According to a SNS Insider, the market size was valued at USD 7.91 billion in 2022 and is projected to reach USD 12.14 billion by 2030, with a compound annual growth rate (CAGR) of 5.5% over the forecast period from 2023 to 2030.Market Scope:
The report provides a comprehensive analysis of the virtual clinical trials market, covering various aspects such as market size, growth drivers, challenges, opportunities, and key trends shaping the industry landscape. It offers insights into different virtual trial models, technology platforms, therapeutic areas, and geographic regions to help stakeholders understand the market dynamics and make informed decisions.
List of Virtual Clinical Trials Companies Profiled in Report:
Clinical Ink Inc.
Dassault Systemes SE
Icon plc
Laboratory Corporation of America Holdings
IQVIA Holdings Inc.
Medable Inc.
Parexel International Corporation
Oracle Corporation
Medpace Holdings Inc.
Wuxi AppTech
Download Free Sample Copy of Virtual Clinical Trials Market Report: https://www.snsinsider.com/sample-request/2493
Market Analysis:
The growth of the virtual clinical trials market is driven by several factors. Traditional clinical trials often face challenges such as patient recruitment and retention, geographical barriers, and high costs and timelines. Virtual trials address these challenges by leveraging digital technologies such as telemedicine, wearable devices, and remote monitoring to conduct clinical research remotely, thereby improving patient access, engagement, and data quality.
Moreover, the COVID-19 pandemic has accelerated the adoption of virtual trial methodologies, as restrictions on in-person interactions highlighted the need for decentralized approaches to clinical research. Pharmaceutical companies, contract research organizations (CROs), and regulatory agencies are increasingly embracing virtual trials as a way to streamline the drug development process, reduce costs, and bring therapies to market more efficiently.
Impact of the Russia-Ukraine Conflict:
The Russia-Ukraine conflict may have implications for the virtual clinical trials market, particularly concerning the conduct of multinational trials and supply chain disruptions. Geopolitical tensions could affect regulatory processes, data privacy laws, and the availability of clinical trial sites and investigators in affected regions. However, the growing trend towards decentralized trial methodologies may mitigate some of these challenges by enabling research to be conducted remotely, independent of geographical constraints.
The Impact of Economic Slowdown:
Economic slowdowns, such as those induced by the COVID-19 pandemic, can impact the virtual clinical trials market by affecting healthcare budgets, research funding, and investor confidence. However, the cost-effectiveness and operational efficiencies offered by virtual trial models make them an attractive option for sponsors and researchers seeking to optimize resources and navigate economic uncertainties. As a result, virtual trials are expected to remain resilient and continue to gain traction in the post-pandemic era.
Virtual Clinical Trials Market Segmentation:
➤ By Study Type
Interventional
Observational
Others
➤ By Phase
Phase 1
Phase 2
Phase 3
Phase 4
➤ By Indication
Oncology
Cardiovascular
Immunology
Gastrointestinal
Respiratory
Endocrinology
Ophthalmology
Ask Your Query Before Buying this Research Report: https://www.snsinsider.com/enquiry/2493
Regional Analysis:
North America and Europe have traditionally been the leading markets for virtual clinical trials, driven by robust healthcare infrastructure, high healthcare expenditure, and a strong focus on advancing medical research. However, the Asia-Pacific region is expected to witness significant growth due to the increasing prevalence of chronic diseases, rising healthcare expenditure, and the adoption of digital technologies in countries like China and India.
Key Takeaways:
➔ The Virtual Clinical Trials Market is projected to experience substantial growth, driven by the increasing demand for decentralized and patient-centric clinical trials, as well as the adoption of digital technologies in medical research.
➔ The Russia-Ukraine conflict has impacted global supply chains, posing challenges for manufacturers operating in the market.
➔ Economic slowdown and budget constraints may influence the adoption rate of virtual clinical trials, particularly in regions with limited resources.
➔ North America and Europe remain leading markets, while the Asia-Pacific region is expected to witness significant growth due to the increasing prevalence of chronic diseases and the adoption of digital technologies.
Recent Developments:
➔ Medidata Solutions, a leading provider of virtual clinical trial solutions, announced a partnership with a major pharmaceutical company to conduct a fully decentralized clinical trial for a rare disease.
➔ Researchers at the University of California, San Francisco, conducted a successful virtual clinical trial for a digital therapeutic intervention for chronic pain management.
➔ The U.S. Food and Drug Administration (FDA) issued guidance on the use of digital health technologies in clinical trials, providing a regulatory framework for the adoption of virtual clinical trial approaches.
Buy Virtual Clinical Trials Market report: https://www.snsinsider.com/checkout/2493
Table of Content
Chapter 1 Introduction
Chapter 2 Research Methodology
Chapter 3 Virtual Clinical Trials Market Dynamics
Chapter 4 Impact Analysis (COVID-19, Ukraine- Russia war, Ongoing Recession on Major Economies)
Chapter 5 Value Chain Analysis
Chapter 6 Porter’s 5 forces model
Chapter 7 PEST Analysis
Chapter 8 Virtual Clinical Trials Market Segmentation, By Study Type
Chapter 9 Virtual Clinical Trials Market Segmentation, By Phase
Chapter 10 Virtual Clinical Trials Market Segmentation, By Indication
Chapter 11 Regional Analysis
Chapter 12 Company profile
Chapter 13 Competitive Landscape
Chapter 14 Use Case and Best Practices
Chapter 15 Conclusion
Continued…
Akash Anand
SNS Insider Pvt. Ltd
+1 415-230-0044
email us here
Visit us on social media:
Facebook
Twitter
LinkedIn
Instagram
YouTube
EIN Presswire does not exercise editorial control over third-party content provided, uploaded, published, or distributed by users of EIN Presswire. We are a distributor, not a publisher, of 3rd party content. Such content may contain the views, opinions, statements, offers, and other material of the respective users, suppliers, participants, or authors.