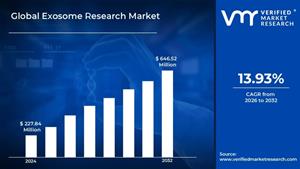
Will Subcutaneous PD-1/PD-L1 Inhibitors Be a Game Changer?
By this fall, the FDA may approve a subcutaneous (SC) injection of the world’s top-selling cancer drug, pembrolizumab (Keytruda; Merck), that can be delivered to patients in 5 minutes or less. It is a change that Merck’s Marjorie Green, MD, senior vice …