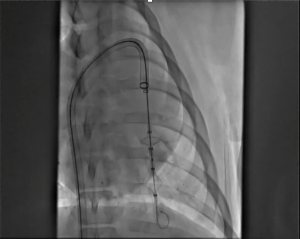
Revolutionizing Thrombectomy: Retriever Medical's DogLeg™ 24F Catheter Study Reveals Remarkable Performance
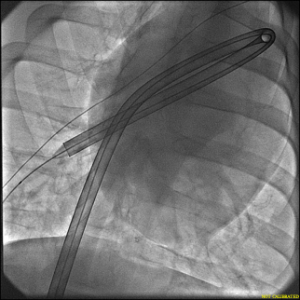
Successful Navigation: The DogLeg 24F Aspiration Catheter effortlessly maneuvers through extreme angles in a porcine model without kinking, ensuring optimal performance in vascular procedures.
Revolutionizing Treatment: Retriever Medical's DogLeg™ 24F Catheter Study Shows Unprecedented Performance in Thrombectomy.
In Vitro and In Vivo Performance Testing of a Novel 24F Aspiration Catheter.
This catheter, designed to navigate tortuous anatomy without compromising inner diameter, has undergone rigorous testing in both in vitro and in vivo settings, resulting in a published study that underscores its exceptional performance.
Led by Dr. Brand Repko, a board-certified Interventional Radiologist and Chief Science Officer at Retriever Medical, Inc., “the study validates the catheter's ability to maintain integrity even in challenging conditions, making it an ideal tool for percutaneous endovascular thrombectomy procedures. This catheter, designed to navigate tortuous anatomy without compromising inner diameter, promises to revolutionize the field of interventional procedures by efficiently removing thrombus and enabling smoother aspiration processes.”
Key findings from the study:
1. Excellent Trackability: The catheter successfully navigated tortuous anatomy and serial 90-180 degree turns without encountering kinking or ovalization.
2. Absence of Distortion: Unlike similar catheters from other manufacturers, the Retriever Medical 24F catheter did not exhibit kinking or ovalization, even at
a radius of curvature of 6mm.
3. Compatibility with Mechanical Thrombectomy Device: The catheter is specifically designed to seamlessly accommodate the advancement of Retriever's
ClotHound ACE Gold PE mechanical thrombectomy device, enabling simultaneous aspiration and mechanical intervention.
“The results of this study reinforce our dedication to delivering cutting-edge solutions to healthcare professionals. Our DogLeg 24F Catheter has shown remarkable promise, laying the groundwork for safer and more effective thrombectomy interventions." - Ben Bobo, CEO of Retriever Medical Inc.
This breakthrough in catheter design holds significant promise for treating conditions such as deep venous thrombosis (DVT) and pulmonary embolism (PE) occlusions. By providing surgeons with a reliable tool that can navigate complex vascular anatomy, Retriever Medical aims to improve patient outcomes and enhance procedural efficiency.
For more information about Retriever Medical and its groundbreaking thrombectomy systems, please visit www.rtvmed.com.
About Retriever Medical: Retriever Medical, Inc. was established with a bold mission to transform interventional medicine by creating groundbreaking surgical solutions, such as the revolutionary ClotHound ACE™ thrombectomy systems. Demonstrating our dedication to innovation, Retriever Medical has significantly expanded its patent portfolio, comprising eight (8) issued U.S. and two (2) international patents, alongside ten (10) pending U.S. patent applications and an additional ten (10) pending foreign patent applications spanning multiple jurisdictions, including the European Community, Hong Kong, Canada, Japan, China, and Mexico. With an unwavering commitment to enhancing patient outcomes and procedural efficiency, Retriever Medical remains at the forefront of driving progress and excellence in healthcare. Retriever Medical's trademarks include Retriever Medical the Retriever Medical logo, ClotHound, ClotHound Blue, ClotHound Gold, ClotHound ACE, Clear ACE, ACE, VORS, and Blood Genie. DogCurve, DogLeg, and DogTail are registered trademarks.
Investors Contact: Ben Bobo Phone: 714.654.2367 Email: bbobo@rtvmed.com
Safe Harbor Statement: This press release includes statements that look forward in time or that express management's beliefs, expectations, or hopes. Such statements are forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements include but are not limited to, the anticipated approval of pending and future patent applications related to Retriever Medical’s ClotHound™, ClotHound ACE™, Clear ACE™, VORS™, and Blood Genie™ Technology, the Company's future patent application filings, and the protection of the Company's intellectual property provided by any patents that issue. These statements are based on current information and beliefs and are not guarantees of future performance. Among the risks and uncertainties that could cause actual results to differ materially from those indicated by such forward-looking statements include that pending and future patent applications related to Retriever Medical’s ClotHound™, ClotHound ACE™, and Blood Genie™ Technology may not result in an issued patent, that the issuance of any patents may be delayed, that the allowed claims, if any, may not be in line with the Company's expectations, that the Company may not be successful in enforcing its patents, and the risk factors detailed from time to time in the Company's periodic Securities and Exchange Commission filings. By making these forward-looking statements, the Company does not undertake to update them in any manner except as may be required by the Company's disclosure obligations in filings it makes with the Securities and Exchange Commission under the federal securities laws.
Ben Bobo
Retriever Medical, Inc.
bbobo@rtvmed.com
Visit us on social media:
Twitter
LinkedIn
EIN Presswire does not exercise editorial control over third-party content provided, uploaded, published, or distributed by users of EIN Presswire. We are a distributor, not a publisher, of 3rd party content. Such content may contain the views, opinions, statements, offers, and other material of the respective users, suppliers, participants, or authors.