Sexual Dimorphism in Cystinuria- The Mitochondria Link
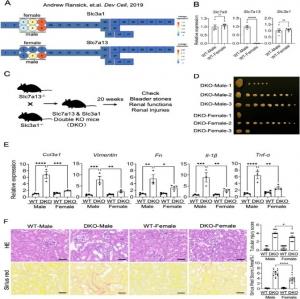
(A) Relative urinary cystine concentration of Slc3a1 KO and WT mice from both sexes. (B) Typical hexagonal cystine crystals from the urine of Slc3a1 KO and WT mice. Scale bar,100 μm. (C) Bladder stones in 20-week-old Slc3a1 KO mice from both sexes. (D) Se
Unravelling the role of mitochondrial Slc3a1 in regulating mitochondrial functions and sexual dimorphism in cystinuria
CHINA, March 28, 2025 /EINPresswire.com/ -- Cystinuria is the most common inheritable cause of kidney stone disease, characterized by impaired reabsorption of cystine and dibasic amino acids in the renal proximal tubules. It exhibits a sex-dependent response, with males experiencing an earlier onset of stone formation and a high number of large-sized stones; however, the cellular origin and mechanisms underlying this sexual dimorphism remains elusive. Recent studies have shown that mitochondrial dysfunction plays a key role in the pathogenesis of renal diseases. It has also been evidenced that there are significant sex-related differences in mitochondrial morphology, function, and homeostasis, as well as variations in response to acute kidney injury and progression of chronic kidney disease.
In a recent study published in the Genes & Diseases journal, researchers from Shanghai Jiao Tong University School of Medicine, East China Normal University, Shanghai University of Traditional Chinese Medicine, Tongji University, and Fudan University unravel the critical role of mitochondrial Slc3a1 in regulating mitochondrial functions and sexual dimorphism in cystinuria.
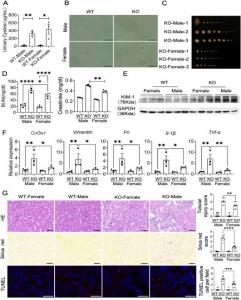
To investigate the mechanisms underlying sexual dimorphism in cystinuria, the authors examined stone formation and kidney injury in Slc3a1 knock-out (KO) mice, Slc3a1, Slc7a13 double KO mice, and orchiectomized Slc3a1 KO mice. The Slc3a1 KO female mice had smaller and less severe bladder stones, concomitant with a lower expression of fibrotic and immune markers than the Slc3a1 KO male mice, showing that cystinuria was more pronounced in males than in females. This severity could not be rescued even upon double KO of Slc3a1 and Slc7a13 or orchidectomy, which establishes that the male susceptibility to cystinuria is dependent on Slc3a1 and independent of Slc7a13.
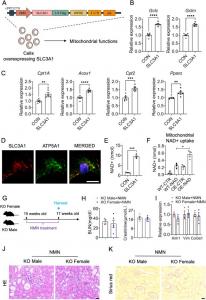
Mitochondrial functions were found to be impaired in the renal tubule cells of Slc3a1 KO male kidneys, resulting in exacerbated damage caused by the accumulating debris and formation of cystine crystal-containing stones; whereas, high SLC3A1 protein levels were associated with enhanced mitochondrial functions in the kidney. By integrating unbiased bulk RNA sequencing, single-cell RNA sequencing, and molecular experiments, the authors showed that i) the differential mitochondrial functions between SLC3A1high male kidneys and SLC3A1low female kidneys primarily arise in the proximal tubule cells; and ii) Slc3a1 enhances mitochondrial functions by increasing mitochondrial NAD+ uptake in the proximal tubules.
In conclusion, this study highlights the critical role of mitochondrial functions in regulating sexual dimorphism in cystinuria. It also suggests that restoring mitochondria in renal tubules of male cystinuria patients may improve mitochondrial function, leading to reduced cell death and attenuation of fibro-inflammation in the renal tubules.
Reference
Title of the original paper - Mitochondrial SLC3A1 regulates sexual dimorphism in cystinuria.
Journal - Genes & Diseases
Genes & Diseases is a journal for molecular and translational medicine. The journal primarily focuses on publishing investigations on the molecular bases and experimental therapeutics of human diseases. Publication formats include full length research article, review article, short communication, correspondence, perspectives, commentary, views on news, and research watch.
DOI - https://doi.org/10.1016/j.gendis.2024.101472
Funding Information:
Science and Technology Commission of Shanghai Municipality of China (No. 23Y21900102; 23ZR1467900 to Q.W.)
Shanghai Rising-Star Program (No. 22QA1405900)
The National Key R&D Program of China (No. 2022YFC2505400, 2022YFC3400203)
The National Natural Science Foundation of China (No. 82100773; 82101486, 82371426)
The Natural Science Foundation of Chongqing, China (No. CSTB2022NSCQ-MSX1621)
The Ningxia Hui Autonomous Region Key Research and Development Project (China) (No. 2022BFH02012)
# # # # # #
Genes & Diseases publishes rigorously peer-reviewed and high quality original articles and authoritative reviews that focus on the molecular bases of human diseases. Emphasis is placed on hypothesis-driven, mechanistic studies relevant to pathogenesis and/or experimental therapeutics of human diseases. The journal has worldwide authorship, and a broad scope in basic and translational biomedical research of molecular biology, molecular genetics, and cell biology, including but not limited to cell proliferation and apoptosis, signal transduction, stem cell biology, developmental biology, gene regulation and epigenetics, cancer biology, immunity and infection, neuroscience, disease-specific animal models, gene and cell-based therapies, and regenerative medicine.
Scopus CiteScore: 7.3 | Impact Factor: 6.9
# # # # # #
More information: https://www.keaipublishing.com/en/journals/genes-and-diseases/
Editorial Board: https://www.keaipublishing.com/en/journals/genes-and-diseases/editorial-board/
All issues and articles in press are available online in ScienceDirect (https://www.sciencedirect.com/journal/genes-and-diseases).
Submissions to Genes & Disease may be made using Editorial Manager (https://www.editorialmanager.com/gendis/default.aspx ).
Print ISSN: 2352-4820
eISSN: 2352-3042
CN: 50-1221/R
Contact Us: editor@genesndiseases.com
X (formerly Twitter): @GenesNDiseases (https://x.com/GenesNDiseases)
Genes & Diseases Editorial Office
Genes & Diseases
+86 23 6571 4691
email us here
Visit us on social media:
Facebook
X
LinkedIn
Instagram
YouTube
Other
Distribution channels: Education, Healthcare & Pharmaceuticals Industry, Science
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release